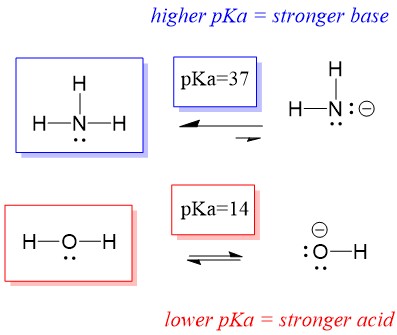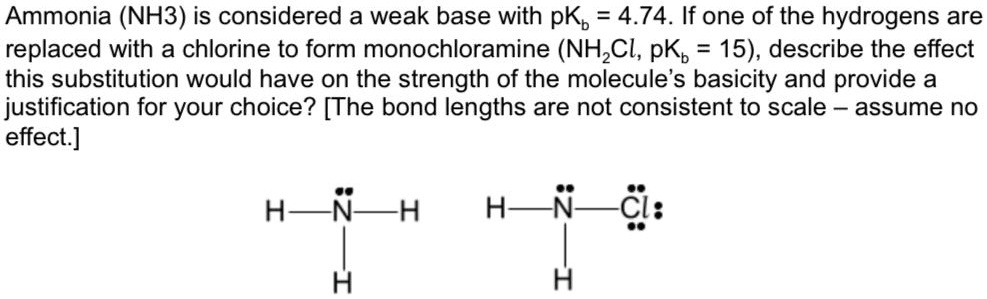
SOLVED: Ammonia (NH3) is considered a weak base with pKb = 4.74. If one of the hydrogens are replaced with a chlorine to form monochloramine (NHZCl, pKb 15) , describe the effect

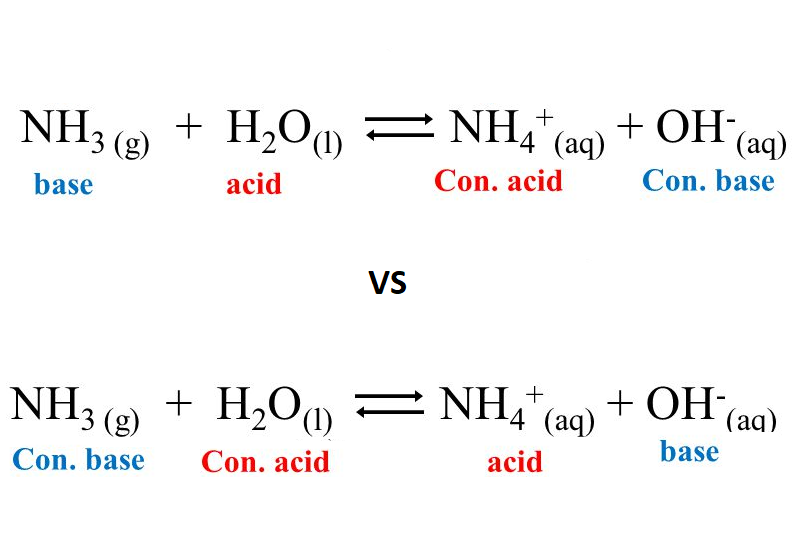
Explain the differences between the Bronsted-Lowry and the Lewis acid-base theories, using the formation of the ammonium ion from ammonia and water to illustrate your points. | Homework.Study.com

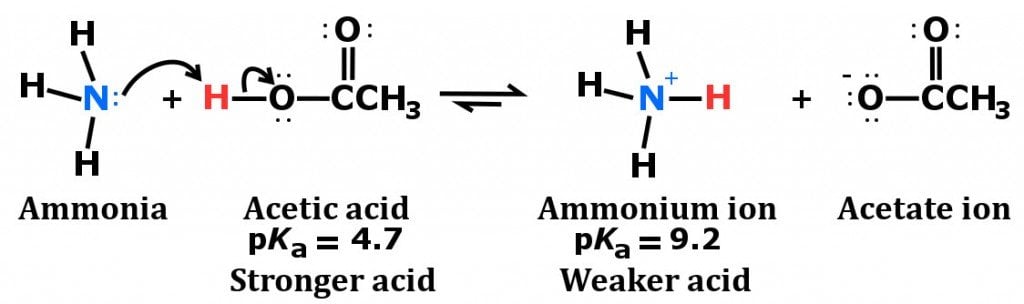
Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram










![Why does \\[N{H_3}\\] act as a Lewis base? Why does \\[N{H_3}\\] act as a Lewis base?](https://www.vedantu.com/question-sets/db470ea0-7477-412c-82d8-a4917a1132145281377342200076057.png)